BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:
URL: http://jsurgery.bums.ac.ir/article-1-159-en.html
Central serous chorioretinopathy treated with topical dorzolamide: A case series study
Gholam Hossain Yaghoobi1, Seyed Abbas Hosseinirad2*, Saeedreza Heydari3
1Professor of Ophthalmology, School of Medicine, Social Determinants of Health Research Center, Birjand University of Medical Sciences, Birjand, Iran
2Assistant Professor of Ophthalmology, School of Medicine, Birjand University of Medical Sciences, Birjand, Iran
3Optometry Student, Mashhad University of Medical Sciences, Mashhad, Iran
Received: June 09, 2018 Revised: December 18, 2018 Accepted: December 18, 2018
|
Abstract The aim of this study was to investigate the improvements of central serous chorioretinopathy (CSC) treated with topical dorzolamide. These observational case studies included nine eyes of the nine patients with CSC, treated by a physician with topical dorzolamide consecutively over a one-month period. The central macular thickness and best-corrected visual acuity (BCVA) were measured and compared with baseline values. All the eyes of nine patients demonstrated subretinal fluid (SRF) before or during the treatment course. The SRF was measured and compared with baseline values in this subgroup. Among the cases (n=9), the mean age was reported as 39±6, and BCVA improved in 77% (7 cases), 11.1% (1 case), and 11.1% (1 case) of the cases within two, four, and ten weeks of treatment after the follow-ups, respectively. Indeed, the mean value of central macular thickness showed improvement from 4.72±1.40 to 3.93±9.8 microns (P=0.211). The SRF decreased during follow-up treatment with the mean time of 0.7±0.6 months; however, central macular thickness, choroidal thickness, and BCVA revealed no significant change. Topical dorzolamide might improve BCVA and decrease SRF in patients with CSC. This case series study demonstrated that topical dorzolamide might be effective in the treatment of CSC. Key words: Central serous chorioretinopathy, Dorzolamide, Following treatment |
Introduction
Chronic central serous chorioretinopathy (CSC) is a vision-threatening disease, characterized by serous accumulation of subretinal fluid (SRF) that causes a localized area of retinal detachment (1-4). Focal laser photocoagulation, photodynamic therapy (PDT), anti-vascular endothelial growth factor agents, corticosteroid inhibition, adrenergic receptor inhibition, and aspirin are considered as the treatment options (1, 5, 6). None of these treatments has been investigated in a large prospective clinical trial. Furthermore, these treatment modalities have variable outcomes (7, 8). The results of performing focal argon laser photocoagulation has been demonstrated to reduce the duration of CSC up to two months, with the complete resolution of retinal pigment epithelium detachment and increased improvement of visual acuity (9-11).
The CSC is a common retinal cause of
visual loss. The mainstays of management are observation, PDT, and laser procedures. Over the past decade, there has been a rapid development in current and novel imaging techniques, functional testing, and CSC management. However, no convincing treatment has been designed for CSC yet (12). According to the literature and the knowledge from the past decade, there is only a single study based on retinal effect of oral acetazolamide. Therefore, this was the first report of topical acetazolamide experience in the treatment of CSC.
Cases
This case series study evaluated the effect of dorzolamide eye drop administered every six h per day during the course of a recent attack (within one week) of CSC in nine patients, including eight male cases and one female case. The mean age of the cases was reported as 39±6.12 years.
The patients underwent complete ophthalmic examination. The diagnosis was established according to biomicroscopic examination with macular oedema observation, fluorescein angiography with the finding of the leakage point (Heidelberg digital system), and optical coherence tomography (OCT) demonstrating the detachment of the macula neuroepithelium.
The inclusion criteria were the patients with the first attack of CSC without any history of operation, laser photocoagulation, or other mimicking retinal diseases. In addition, the exclusion criteria were non-cooperative cases and patients with allergy to fluorescein, the subjects with contraindications to the administration of acetazolamide, or those not present in the follow-up examinations. The patients who did not respond to treatment within two and a half months of intervention and did not agree to the continuation of conservative therapy underwent other therapeutic approaches. For the purpose of statistical comparison, t-test and SPSS software (version 18) were used and P-value was considered statistically significant (0.05).
The average BCVAs of nine patients in the study group were 20/40 and 20/36 before and after the treatment, respectively. The mean time of follow-up treatment (i.e., acetazolamide eye drop instillation) was 0.7±0.6 months. The average duration of the neuroepithelium detachment in this group was four weeks. The shortest and longest period of neuroepithelium was one week and four weeks, respectively. The mean age of the cases was 39±6; the BCVA improved in 77% (7 cases), 11.1% (1 case), and 11.1% (1 case) of the cases within two, four, and ten weeks of treatment after the follow-ups, respectively. Indeed, the mean value
of central macular thickness showed an improvement from 472±1.40 to 393±9.8 microns (P=0.211) (Figures 1 and Figure 2). The subretinal fluid was decreased during the mean time of 0.7±0.6 month in the follow-up treatment; however, this reduction in central macular thickness and improved BCVA demonstrated no statistically significant difference.
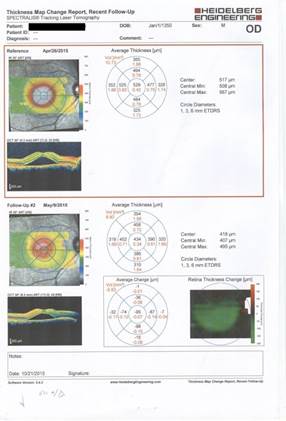
Figure 1: Treatment response during follow-up
Discussion
Topical dorzolamide might improve BCVA and decrease subretinal fluid in patients with CSC. This case series study demonstrated that topical dorzolamide might be effective in the treatment of CSC. According to the results of many studies it was revealed that it is usually self-limited; however, the need for rapid visual recovery may prompt treatment with photodynamic therapy, antimineralocorticoid, rifampin, and ketoconazole (12-15). Khan et al. reported a case of bilateral CSC that was treated with focal laser therapy. After two years, the relapse occurred and was treated successfully with oral acetazolamide. Moreover, the second relapse happened after one year and the related re-evaluation of the patient and antituberculosis therapy were successful (16).
Esfihani et al. showed that oral ketoconazole may be a non-invasive, safe, and effective therapeutic option regarding the patients with
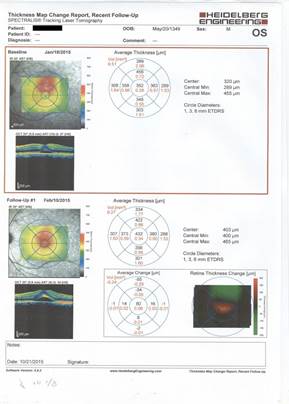
Figure 2: Treatment response following treatment
acute CSC (17). There is also report of CSC in a patient treated by isotretinoin (18). Based on the mentioned findings it was revealed that the etiology of this disease is multifactorial and several treatment modalities could be considered for the cases. In addition, the previously mentioned results indicate the role of topical carbonic anhydrase inhibitor in the treatment of CSC, despite the fact that the long-term follow-up failed, which might be due to failed or recovered patient visual acuity. In a study carried out by Virgilio Lima-Gómez et al. on the efficacy of dorzolamide to reduce retinal thickness following photocoagulation in diabetic macular oedema, the improvements were observed in the reduction of retinal thickness (19).
In a single study, the patients treated with
oral acetazolamide showed faster subjective improvements and SRF resorption; furthermore, the mean values of resolution time were 3.3±1.1 and 7.7±1.5 weeks in the treatment and control groups, respectively (P=0.0001). There was no significant difference in final visual acuity or relapse rates between the two groups (20). The CSC is incompletely understood, though the improvements in imaging technology have led to further comprehension of the disease management. Up to now, PDT has demonstrated satisfactory clinical results; however, focal laser and PDT are the present standard treatments regarding CSC. Nonetheless, all cases did not show any improvements in the course of therapeutic approach. Consequently, further studies are needed to detect appropriate treatment considering CSC.
Conclusions
This single study in patients treated with oral acetazolamide demonstrated faster subjective improvements and SRF resorption. Topical dorzolamide might improve BCVA and decrease SRF in patients with CSC. This case series study demonstrated that topical dorzolamide might be effective in the treatment of CSC.
Acknowledgments
The authors would like to thank all the patients for cooperation in this project.
Conflict of Interest
The authors declare that there is no conflict of interest.
References
1. Singh RP, Sears JE, Bedi R, Schachat AP, Ehlers JP, Kaiser PK. Oral eplerenone for the management of chronic central serous chorioretinopathy. Int J Ophthalmol. 2015; 8(2):310-4. PMID: 25938046 DOI: 10.3980/j.issn.2222-3959.2015.02.17
2. Caccavale A, Imparato M, Romanazzi F, Negri A, Porta A, Ferentini F. A new strategy of treatment with low-dosage acetyl salicylic acid in patients affected by central serous chorioretinopathy. Med Hypotheses. 2009; 73(3):435-7. PMID: 19427737 DOI: 10.1016/j.mehy.2009.03.036
3. Daruich A, Matet A, Marchionno L, De Azevedo JD, Ambresin A, Mantel I, et al. Acute central serous chorioretinopathy: factors influencing episode duration. Retina. 2017; 37(10):1905-15. PMID: 28067724 DOI: 10.1097/IAE.0000000000001443
4. Lin HY, Peng KL. Bilateral Chronic Central Serous Chorioretinopathy (CSC) induced by long-term testosterone treatment. J Clin Exp Ophthalmol. 2015; 6:509. doi:10.4172/2155-9570.1000509
5. Chin EK, Almeida DR, Roybal CN, Niles PI, Gehrs KM, Sohn EH, et al. Oral mineralocorticoid antagonists for recalcitrant central serous chorioretinopathy. Clin Ophthalmol. 2015; 9:1449-56. PMID: 26316684 DOI: 10.2147/OPTH.S86778
6. Al Mamoori F. IQ 532 Micropulse green laser treatment for refractory chronic central serous retinopathy. EC Ophthalmol. 2017; 6(1):25-9.
7. Cebeci Z, Oray M, Bayraktar S, Tuğal-Tutkun I, Kır N. Atypical central serous chorioretinopathy..Turk J Ophthalmol. 2017; 47(4):238-42. PMID: 28845331 DOI: 10.4274/tjo.38039
8. Das S. Central serous chorioretinopathy (CSC). Med Vision Res Found. 2017; 35(3):10-20.
9. Yang D, Eliott D. Systemic mineralocorticoid antagonists in the treatment of central serous chorioretinopathy. J Semin Ophthalmol. 2017; 32(1):36-42. PMID: 27929707 DOI: 10.1080/
08820538.2016.1228418
10. Ross A, Ross AH, Mohamed Q. Review and update of central serous chorioretinopathy. Curr Opin Ophthalmol. 2011; 22(3):166-73. PMID: 21427570 DOI: 10.1097/ICU.0b013e3283459826
11. Liew G, Quin G, Gillies M, Fraser-Bell S. Central serous chorioretinopathy: a review of epidemiology and pathophysiology. Clin Exp Ophthalmol. 2013; 41(2):201-14. PMID: 22788735 DOI: 10.1111/j.
1442-9071.2012.02848.x
12. Wong KH, Lau KP, Chhablani J, Tao Y, Li Q, Wong IY. Central serous chorioretinopathy: what we have learnt so far. Acta Ophthalmol. 2016; 94(4):321-5. PMID: 26132864 DOI: 10.1111/aos.12779
13. Iacono P, Battaglia Parodi M, Falcomata B, Bandello F. Central serous chorioretionpathy treatments: a mini review. Ophthalamic Res. 2015; 55(2):76-83. PMID: 26619293 DOI: 10.1159/000441502
14. Do JL, Olmos de Koo LC, Ameri H. Atypical chronic central serous chorioretinopathy with cystoid macular edema: therapeutic response to medical and laser therapy. J Curr Ophthalmol. 2017; 29(2):133-5. PMID: 28626824 DOI: 10.1016/j.joco.2017.01.004
15. Dave PA, Chhablani J. Oral eplerenone for treatment of bullous retinal detachment secondary to chronic central serous retinopathy. Kerala J Ophthalmol. 2017; 29(1):54-6. DOI: 10.4103/kjo.kjo_39_17
16. Khan P, Khan L, Anjum N, Saxena N. Central serous chorioretinopathy secondary to tuberculosis: cause or coincidence. BMJ Case Rep. 2017; 2017:216471. PMID: 28500259 DOI: 10.1136/bcr-2016-216471
17. Esfahani MR, Torabi HR, Harandi ZA, Zarei M. Ketoconazole in the treatment of central serous chorioretinopathy. Iran J Ophthalmol. 2010; 22(4):59.
18. Buyuktortop N, Onaran Z, ÖzkalF, Yumusak E. Central serous chorioretinopathy probably associated
with isotretinoin in a keratoconus patient. Can J Ophthalmol. 2017; 53(4):e162-5. PMID: 30119816 DOI: 10.1016/j.jcjo.2017.10.032
19. Lima-Gomez V, Bermudez-Zapata DA, Blanco-Hernandez DM. Efficacy of dorzolamide in reducing retinal thickness after photocoagulation in diabetic macular oedema. Cir Cir. 2015; 83(1):3-8. PMID: 25982601 DOI: 10.1016/j.circir.2014.08.001
20. Nicholson B, Noble J, Forooghian F, Meyerle C. Central serous chorioretinopathy: update on pathophysiology and treatment. Surv Ophthalmol. 2013; 58(2):103-26. PMID: 23410821 DOI: 10.1016/j.survophthal.2012.07.004
Received: 2018/06/9 | Accepted: 2018/12/18 | ePublished ahead of print: 2019/03/11 | Published: 2019/02/24 | ePublished: 2019/02/24
| Rights and permissions | |
 |
This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License. |







