Volume 13, Issue 3 (9-2025)
J Surg Trauma 2025, 13(3): 90-95 |
Back to browse issues page
Download citation:
BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:



BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:
Ghaemi M, Kheradmand D. Cancer-Associated Thrombosis in Brain Tumors: Challenges in Diagnosis and Management. J Surg Trauma 2025; 13 (3) :90-95
URL: http://jsurgery.bums.ac.ir/article-1-470-en.html
URL: http://jsurgery.bums.ac.ir/article-1-470-en.html
Department of Neurosurgery, School of Medicine, Birjand University of Medical Sciences, Birjand, Iran
Full-Text [PDF 398 kb]
(609 Downloads)
| Abstract (HTML) (1551 Views)
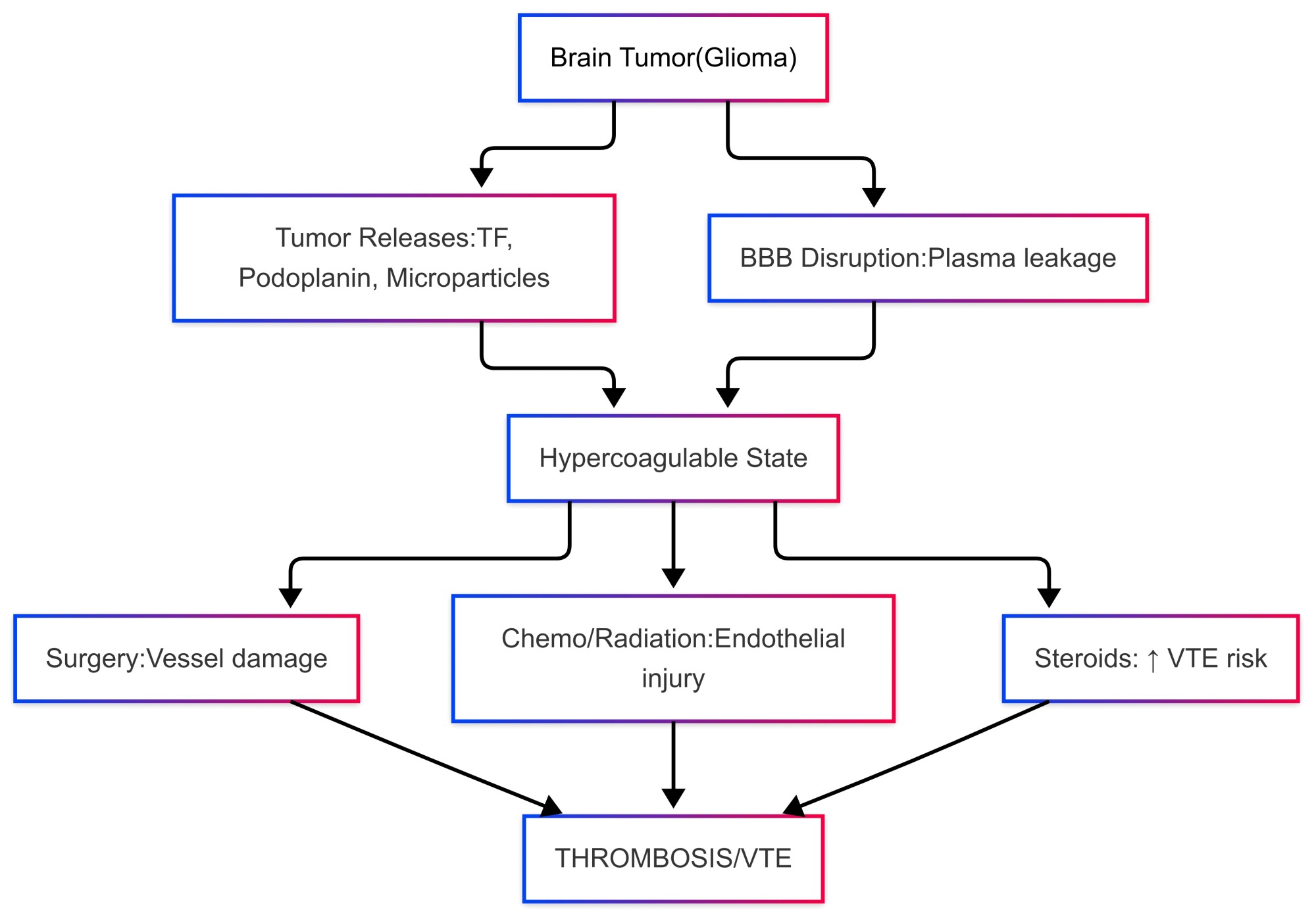
Table 1. Comparison of anticoagulants in brain tumor patients. LMWH = Low-molecular-weight heparin; DOACs = Direct oral anticoagulants; ICH = Intracranial hemorrhage.
Prophylaxis
Primary thromboprophylaxis is advised for patients with high-risk brain tumors, especially during the perioperative period. The LMWH is the preferred option due to its favorable safety profile and effectiveness in reducing the incidence of VTE. However, routine thromboprophylaxis outside of the perioperative period is not universally recommended, as there is insufficient evidence and potential risks of bleeding associated with its use (1. 14, 15).
Management of Complications
When managing ICH in patients who are on anticoagulants, swift action is essential. The first step is to stop the anticoagulation immediately. It is important to administer reversal agents right away, such as protamine sulfate for heparin or vitamin K for warfarin. Additionally, consulting a neurosurgeon is crucial in cases of significant hemorrhages. When considering whether to restart anticoagulation after an ICH, the decision must be carefully customized for each patient, weighing their risk of thrombosis against the stability of the hemorrhage (8, 21).
Prognostic Implications
The VTE has a significant impact on the prognosis of patients with brain tumors, especially those diagnosed with high-grade gliomas. The presence of VTE is linked to a higher risk of mortality, with glioblastoma patients showing an adjusted hazard ratio of 1.3. This increased mortality risk stems from complications, such as pulmonary embolism, recurring thrombotic events, and the complexities of anticoagulation management in light of a heightened risk for ICH. Furthermore, VTE negatively affects patients' quality of life by worsening symptoms like pain, swelling, and shortness of breath. Long-term anticoagulation therapy adds another layer of difficulty to patient management, bringing its own set of risks and monitoring demands, which can heighten anxiety and diminish overall functional status (5-7).
Future Directions
Addressing the challenges of VTE in patients with brain tumors demands progress in several key areas, such as risk assessment, new anticoagulants, biomarker discovery, and personalized medicine. The development of sophisticated risk assessment tools that combine clinical, genetic, and molecular markers is crucial for pinpointing high-risk patients. For instance, isocitrate dehydrogenase (IDH) mutations found in gliomas have been linked to a reduced risk of VTE, indicating that genetic profiling could play a significant role in improving risk assessment (22). The emergence of DOACs, like rivaroxaban and apixaban, offers potential benefits compared to traditional anticoagulants, such as LMWH. While emerging data suggest that DOACs may present a favorable safety profile for brain tumor patients, further studies are necessary to verify their effectiveness and safety within this population. Additionally, investigational anticoagulants that target factor XI are currently being examined for their ability to prevent thrombosis without significantly impacting hemostasis (19, 23). Identifying reliable biomarkers for assessing VTE risk in brain tumor patients remains an active area of research. Potential biomarkers, including TF, podoplanin, and circulating microparticles, are being explored for their ability to predict thrombotic events. The aim is to create a panel of biomarkers that can be used in conjunction with clinical risk factors to enhance the accuracy of VTE predictions (24).
Research in personalized medicine focuses on tailoring anticoagulation therapy to fit individual patient risk profiles. This approach involves using genetic and molecular data to inform treatment decisions, such as selecting the appropriate anticoagulant and determining the length of therapy. The objective of personalized treatment plans is to strike the right balance between reducing thrombotic risk and minimizing the likelihood of bleeding complications, especially intracerebral hemorrhages (25). Additionally, artificial intelligence (AI) holds great promise in enhancing our understanding of thrombosis risk. By meticulously analyzing extensive datasets that include patient demographics, detailed tumor characteristics, and a comprehensive range of biomarker profiles, AI can uncover valuable insights. This cutting-edge methodology enables more accurate identification of individuals at elevated risk for thrombosis, revolutionizing how healthcare professionals evaluate and manage these risks.
The present review had significant limitations. First, the heterogeneity across studies, such as variations in tumor types, grades, and anticoagulation regimens, prevented direct comparisons and meta-analysis. Second, the evidence base for DOACs in this population was limited, with few RCTs available. Additionally, potential publication bias might have affected our findings, as negative studies were often underrepresented, and excluding non-English literature might have omitted relevant data. Finally, the fast-evolving landscape of anticoagulant therapies and biomarkers highlights the need for timely reevaluation and standardized, prospective studies to improve thrombosis management in neuro-oncology.
Conclusions
The CAT presents significant challenges for patients with brain tumors influenced by tumor biology, treatment complications, and immobility. The diagnosis of CAT is difficult due to symptom overlap and bleeding risks. The LMWHs are the primary treatment, although DOACs and personalized approaches may improve outcomes. Effective management requires collaboration among oncologists, neurologists, and hematologists for risk assessments and tailored therapies. The present review summarizes current knowledge of CAT in brain tumor patients, emphasizing the need for more research into the efficacy and safety of DOACs, as well as demographic factors influencing VTE risk. A holistic, evidence-based approach is essential for minimizing thrombotic and bleeding complications while enhancing quality of life. In addition, future research and multidisciplinary collaborations are imperative for achieving these objectives.
Conflict of Interest
The authors declare that they have no competing interests.
Full-Text: (308 Views)
Abstract
Cancer-associated thrombosis (CAT) represents a significant challenge in patients with brain tumors, with venous thromboembolism (VTE) affecting up to 30% of those with high-grade gliomas and 20% of those with brain metastases or primary CNS lymphoma. The primary clinical dilemma lies in balancing the prevention of thrombosis with the heightened risk of intracranial hemorrhage (ICH). The heightened risk of VTE is driven by tumor-induced hypercoagulability and the effects of cancer treatments. The present literature review evaluated evidence from PubMed/MEDLINE and Web of Science. Clinical studies, mechanistic research, and guidelines (ASCO/NCCN) were prioritized, while case reports and non-English studies were excluded. Diagnosing VTE in brain tumor patients is complicated by overlapping symptoms with tumor progression and treatment side effects, as well as limitations in imaging and biomarker tests. Effective management of CAT requires balancing the prevention of thrombosis with the minimization of ICH risks. Low-molecular-weight heparin (LMWH) remains the preferred anticoagulant, although direct oral anticoagulants (DOACs) are emerging as potential alternatives. While prophylactic anticoagulation is recommended in high-risk perioperative settings, its routine use in other contexts remains controversial due to bleeding risks. The VTE significantly impacts survival and quality of life, increasing mortality and symptom burden. Future efforts should focus on improving risk stratification, exploring novel anticoagulants, and personalizing treatment plans. Additionally, the limitations of existing studies, such as the lack of robust data on DOACs in this population, must be addressed. Multidisciplinary collaboration and ongoing research are crucial for advancing the prevention and treatment of CAT in patients with brain tumors.
Keywords: Anticoagulants, Brain Neoplasms, Heparin, Venous Thromboembolism, Warfarin
Cancer-associated thrombosis (CAT) represents a significant challenge in patients with brain tumors, with venous thromboembolism (VTE) affecting up to 30% of those with high-grade gliomas and 20% of those with brain metastases or primary CNS lymphoma. The primary clinical dilemma lies in balancing the prevention of thrombosis with the heightened risk of intracranial hemorrhage (ICH). The heightened risk of VTE is driven by tumor-induced hypercoagulability and the effects of cancer treatments. The present literature review evaluated evidence from PubMed/MEDLINE and Web of Science. Clinical studies, mechanistic research, and guidelines (ASCO/NCCN) were prioritized, while case reports and non-English studies were excluded. Diagnosing VTE in brain tumor patients is complicated by overlapping symptoms with tumor progression and treatment side effects, as well as limitations in imaging and biomarker tests. Effective management of CAT requires balancing the prevention of thrombosis with the minimization of ICH risks. Low-molecular-weight heparin (LMWH) remains the preferred anticoagulant, although direct oral anticoagulants (DOACs) are emerging as potential alternatives. While prophylactic anticoagulation is recommended in high-risk perioperative settings, its routine use in other contexts remains controversial due to bleeding risks. The VTE significantly impacts survival and quality of life, increasing mortality and symptom burden. Future efforts should focus on improving risk stratification, exploring novel anticoagulants, and personalizing treatment plans. Additionally, the limitations of existing studies, such as the lack of robust data on DOACs in this population, must be addressed. Multidisciplinary collaboration and ongoing research are crucial for advancing the prevention and treatment of CAT in patients with brain tumors.
Keywords: Anticoagulants, Brain Neoplasms, Heparin, Venous Thromboembolism, Warfarin
Introduction
Patients with brain tumors face a significantly increased risk of developing venous thromboembolism (VTE), a serious condition characterized by blood clots in the veins. The prevalence of VTE in this population can reach as high as 30% in cases of high-grade gliomas and 20% in individuals with brain metastases or primary CNS lymphoma. This tendency for tumor-induced hypercoagulability stems from the overproduction of pro-coagulant proteins, such as tissue factor (TF) and podoplanin, which facilitate the clotting process (1-4). The clinical management of VTE in patients with brain tumors is particularly challenging due to the simultaneous risks of thrombosis and intracranial hemorrhage (ICH), making treatment decisions more complicated. The VTE can negatively impact both survival rates and quality of life, with several factors influencing the heightened risk of clot formation. These factors include the type of tumor, its grade, recent surgical procedures, and specific cancer treatments. Additionally, managing VTE in these patients is complicated by the increased risk of ICH associated with anticoagulation therapy (5-7).
Despite the potential risks, therapeutic anticoagulation continues to be the standard approach for addressing VTE. Low-molecular-weight heparin (LMWH) is typically favored due to its favorable safety profile, although direct oral anticoagulants (DOACs) are emerging as possible alternatives. However, their efficacy and safety in patients with brain tumors still require further investigation (6-8).
The present review aims to explore the complex pathophysiology of cancer-associated thrombosis (CAT), specifically in brain tumor patients. It examines the challenges associated with diagnosing CAT and investigates current management strategies for this condition. Furthermore, the review discusses future directions for improving outcomes and quality of life within this patient population. To gather evidence, we conducted a thorough search of PubMed/MEDLINE and Web of Science using search terms such as "brain tumor thrombosis" and "glioblastoma coagulopathy." We prioritized clinical studies, including cohort studies and randomized controlled trials (RCTs), as well as seminal mechanistic papers with 50 or more citations and current guidelines from ASCO and NCCN. However, we excluded case reports, non-English publications, and studies lacking histopathological confirmation.
Methods
The present literature review provides an analytical evaluation of current evidence regarding CAT in patients with brain tumors through a comprehensive literature search. The PubMed/MEDLINE and Web of Science databases were queried using keywords, such as "brain tumor thrombosis," "glioblastoma coagulopathy," and "VTE in brain neoplasms." Inclusion criteria focused on clinical studies (e.g., cohort studies, RCTs), seminal mechanistic papers (≥50 citations), and guidelines from ASCO and NCCN. Case reports, non-English publications, and studies lacking histopathological confirmation were excluded. Data were analyzed thematically, concentrating on pathophysiology, diagnostic challenges, management strategies (e.g., LMWH, DOACs, warfarin), and prognostic implications.
Results
Pathophysiology of Thrombosis in Brain Tumors
The development of thrombosis in brain tumors is a complex and multifaceted process influenced by several factors. Tumor-derived pro-coagulant substances play a significant role in this phenomenon, alongside the activation of the coagulation pathway, platelet aggregation, and treatment-related variables. Glioma cells, in particular, produce pro-coagulant substances like TF and podoplanin, which initiate the clotting cascade and facilitate platelet aggregation. These tumor cells also release microparticles rich in TF, further amplifying the coagulation process (9-12).
In patients with brain tumors, the integrity of the blood-brain barrier is frequently compromised, allowing for the leakage of plasma proteins and pro-coagulant factors into the brain tissue. This disruption fosters local thrombus formation and contributes to a hypercoagulable state in the affected regions (9). Furthermore, various treatment modalities, including surgery, chemotherapy, radiation therapy, and corticosteroids, can escalate the risk of thrombotic events. Surgical procedures may directly damage blood vessels, while chemotherapy and radiation can lead to endothelial injury and inflammation. Additionally, corticosteroids, often used to alleviate cerebral edema, may induce a hypercoagulable state, further increasing the risk of VTE (Figure 1) (13).
Clinical Challenges in Diagnosis
The diagnosis of VTE in brain tumor patients is clinically challenging due to several factors. Symptoms like headaches, confusion, and focal neurological deficits can overlap with tumor progression or treatment side effects, complicating clinical assessment and potentially delaying diagnosis. Imaging techniques like CT or MRI, while essential for diagnosis, may have limitations in brain tumor patients due to tumor-related changes that can obscure or mimic thrombotic events (2, 14, 15).
Patients with brain tumors face a significantly increased risk of developing venous thromboembolism (VTE), a serious condition characterized by blood clots in the veins. The prevalence of VTE in this population can reach as high as 30% in cases of high-grade gliomas and 20% in individuals with brain metastases or primary CNS lymphoma. This tendency for tumor-induced hypercoagulability stems from the overproduction of pro-coagulant proteins, such as tissue factor (TF) and podoplanin, which facilitate the clotting process (1-4). The clinical management of VTE in patients with brain tumors is particularly challenging due to the simultaneous risks of thrombosis and intracranial hemorrhage (ICH), making treatment decisions more complicated. The VTE can negatively impact both survival rates and quality of life, with several factors influencing the heightened risk of clot formation. These factors include the type of tumor, its grade, recent surgical procedures, and specific cancer treatments. Additionally, managing VTE in these patients is complicated by the increased risk of ICH associated with anticoagulation therapy (5-7).
Despite the potential risks, therapeutic anticoagulation continues to be the standard approach for addressing VTE. Low-molecular-weight heparin (LMWH) is typically favored due to its favorable safety profile, although direct oral anticoagulants (DOACs) are emerging as possible alternatives. However, their efficacy and safety in patients with brain tumors still require further investigation (6-8).
The present review aims to explore the complex pathophysiology of cancer-associated thrombosis (CAT), specifically in brain tumor patients. It examines the challenges associated with diagnosing CAT and investigates current management strategies for this condition. Furthermore, the review discusses future directions for improving outcomes and quality of life within this patient population. To gather evidence, we conducted a thorough search of PubMed/MEDLINE and Web of Science using search terms such as "brain tumor thrombosis" and "glioblastoma coagulopathy." We prioritized clinical studies, including cohort studies and randomized controlled trials (RCTs), as well as seminal mechanistic papers with 50 or more citations and current guidelines from ASCO and NCCN. However, we excluded case reports, non-English publications, and studies lacking histopathological confirmation.
Methods
The present literature review provides an analytical evaluation of current evidence regarding CAT in patients with brain tumors through a comprehensive literature search. The PubMed/MEDLINE and Web of Science databases were queried using keywords, such as "brain tumor thrombosis," "glioblastoma coagulopathy," and "VTE in brain neoplasms." Inclusion criteria focused on clinical studies (e.g., cohort studies, RCTs), seminal mechanistic papers (≥50 citations), and guidelines from ASCO and NCCN. Case reports, non-English publications, and studies lacking histopathological confirmation were excluded. Data were analyzed thematically, concentrating on pathophysiology, diagnostic challenges, management strategies (e.g., LMWH, DOACs, warfarin), and prognostic implications.
Results
Pathophysiology of Thrombosis in Brain Tumors
The development of thrombosis in brain tumors is a complex and multifaceted process influenced by several factors. Tumor-derived pro-coagulant substances play a significant role in this phenomenon, alongside the activation of the coagulation pathway, platelet aggregation, and treatment-related variables. Glioma cells, in particular, produce pro-coagulant substances like TF and podoplanin, which initiate the clotting cascade and facilitate platelet aggregation. These tumor cells also release microparticles rich in TF, further amplifying the coagulation process (9-12).
In patients with brain tumors, the integrity of the blood-brain barrier is frequently compromised, allowing for the leakage of plasma proteins and pro-coagulant factors into the brain tissue. This disruption fosters local thrombus formation and contributes to a hypercoagulable state in the affected regions (9). Furthermore, various treatment modalities, including surgery, chemotherapy, radiation therapy, and corticosteroids, can escalate the risk of thrombotic events. Surgical procedures may directly damage blood vessels, while chemotherapy and radiation can lead to endothelial injury and inflammation. Additionally, corticosteroids, often used to alleviate cerebral edema, may induce a hypercoagulable state, further increasing the risk of VTE (Figure 1) (13).
Clinical Challenges in Diagnosis
The diagnosis of VTE in brain tumor patients is clinically challenging due to several factors. Symptoms like headaches, confusion, and focal neurological deficits can overlap with tumor progression or treatment side effects, complicating clinical assessment and potentially delaying diagnosis. Imaging techniques like CT or MRI, while essential for diagnosis, may have limitations in brain tumor patients due to tumor-related changes that can obscure or mimic thrombotic events (2, 14, 15).

Figure 1. Pathophysiological mechanisms of thrombosis in brain tumors
The use of D-dimer as a biomarker for VTE diagnosis is also problematic in this population. Elevated D-dimer levels are common in cancer patients due to the hypercoagulable state associated with malignancy, reducing its diagnostic specificity.
Leveraging a diverse array of alternative strategies, particularly the integration of multiple biomarkers, such as TF, podoplanin, and circulating microparticles, has the potential to improve diagnostic accuracy significantly. Furthermore, factors associated with treatment, including surgical interventions, chemotherapy, and radiation therapy, can elevate the risk of VTE and may manifest symptoms that closely resemble those of thrombotic events, thereby complicating clinical assessment (16, 17).
Management Strategies
Anticoagulation Therapy
In the management of VTE in patients with brain tumors, low LMWH stands out as the preferred treatment option. This preference is primarily due to its demonstrated efficacy and a favorable safety profile, particularly in terms of a reduced risk of ICH when compared to warfarin. Research indicates that LMWHs effectively prevent recurrent VTE events, supporting their use in this patient population (15, 18). In recent years, direct oral DOACs have emerged as a promising alternative to LMWHs. Some evidence suggests that DOACs may exhibit a lower risk of major bleeding and ICH; however, data specific to brain tumor patients remain limited, necessitating a careful assessment of bleeding risks before initiating DOAC therapy (19, 8). On the other hand, warfarin is generally considered less favorable in this context due to its requirement for regular INR monitoring, the potential for drug interactions, and dietary restrictions that complicate anticoagulation management in patients with brain tumors (Table 1) (1, 15).
Timing of Anticoagulation
Striking a careful balance between the risk of thrombosis and the potential for bleeding complications, especially in the delicate postoperative environment, is of paramount importance. Administering prophylactic anticoagulation within the first 24 h following surgical procedures is recommended, as this approach has been shown to substantially decrease the likelihood of thromboembolic events while minimizing the risk of major bleeding complications. However, for patients identified as being at elevated risk for intracerebral hemorrhage, the initiation of anticoagulation therapy may need to be postponed. In such cases, this decision must be made with close monitoring to ensure patient safety (20).
Leveraging a diverse array of alternative strategies, particularly the integration of multiple biomarkers, such as TF, podoplanin, and circulating microparticles, has the potential to improve diagnostic accuracy significantly. Furthermore, factors associated with treatment, including surgical interventions, chemotherapy, and radiation therapy, can elevate the risk of VTE and may manifest symptoms that closely resemble those of thrombotic events, thereby complicating clinical assessment (16, 17).
Management Strategies
Anticoagulation Therapy
In the management of VTE in patients with brain tumors, low LMWH stands out as the preferred treatment option. This preference is primarily due to its demonstrated efficacy and a favorable safety profile, particularly in terms of a reduced risk of ICH when compared to warfarin. Research indicates that LMWHs effectively prevent recurrent VTE events, supporting their use in this patient population (15, 18). In recent years, direct oral DOACs have emerged as a promising alternative to LMWHs. Some evidence suggests that DOACs may exhibit a lower risk of major bleeding and ICH; however, data specific to brain tumor patients remain limited, necessitating a careful assessment of bleeding risks before initiating DOAC therapy (19, 8). On the other hand, warfarin is generally considered less favorable in this context due to its requirement for regular INR monitoring, the potential for drug interactions, and dietary restrictions that complicate anticoagulation management in patients with brain tumors (Table 1) (1, 15).
Timing of Anticoagulation
Striking a careful balance between the risk of thrombosis and the potential for bleeding complications, especially in the delicate postoperative environment, is of paramount importance. Administering prophylactic anticoagulation within the first 24 h following surgical procedures is recommended, as this approach has been shown to substantially decrease the likelihood of thromboembolic events while minimizing the risk of major bleeding complications. However, for patients identified as being at elevated risk for intracerebral hemorrhage, the initiation of anticoagulation therapy may need to be postponed. In such cases, this decision must be made with close monitoring to ensure patient safety (20).
Table 1. Comparison of anticoagulants in brain tumor patients. LMWH = Low-molecular-weight heparin; DOACs = Direct oral anticoagulants; ICH = Intracranial hemorrhage.
| Anticoagulant | Efficacy in VTE Prevention | Safety (Bleeding/ICH Risk) | Monitoring Requirements | Key Considerations |
| LMWH (e.g., enoxaparin) | High (1st-line for CAT) | Lower ICH risk vs. warfarin | Weekly anti-Xa (optional) | Preferred in perioperative settings; renal dose adjustment needed. |
| DOACs (e.g., apixaban) | Emerging evidence for efficacy | Potentially lower major bleeding risk | Minimal (no routine labs) | Limited data in brain tumors; avoided in severe renal impairment. |
| Warfarin | Moderate (historically used) | Higher ICH risk | Frequent INR monitoring | Drug/diet interactions; less favorable in this population. |
Prophylaxis
Primary thromboprophylaxis is advised for patients with high-risk brain tumors, especially during the perioperative period. The LMWH is the preferred option due to its favorable safety profile and effectiveness in reducing the incidence of VTE. However, routine thromboprophylaxis outside of the perioperative period is not universally recommended, as there is insufficient evidence and potential risks of bleeding associated with its use (1. 14, 15).
Management of Complications
When managing ICH in patients who are on anticoagulants, swift action is essential. The first step is to stop the anticoagulation immediately. It is important to administer reversal agents right away, such as protamine sulfate for heparin or vitamin K for warfarin. Additionally, consulting a neurosurgeon is crucial in cases of significant hemorrhages. When considering whether to restart anticoagulation after an ICH, the decision must be carefully customized for each patient, weighing their risk of thrombosis against the stability of the hemorrhage (8, 21).
Prognostic Implications
The VTE has a significant impact on the prognosis of patients with brain tumors, especially those diagnosed with high-grade gliomas. The presence of VTE is linked to a higher risk of mortality, with glioblastoma patients showing an adjusted hazard ratio of 1.3. This increased mortality risk stems from complications, such as pulmonary embolism, recurring thrombotic events, and the complexities of anticoagulation management in light of a heightened risk for ICH. Furthermore, VTE negatively affects patients' quality of life by worsening symptoms like pain, swelling, and shortness of breath. Long-term anticoagulation therapy adds another layer of difficulty to patient management, bringing its own set of risks and monitoring demands, which can heighten anxiety and diminish overall functional status (5-7).
Future Directions
Addressing the challenges of VTE in patients with brain tumors demands progress in several key areas, such as risk assessment, new anticoagulants, biomarker discovery, and personalized medicine. The development of sophisticated risk assessment tools that combine clinical, genetic, and molecular markers is crucial for pinpointing high-risk patients. For instance, isocitrate dehydrogenase (IDH) mutations found in gliomas have been linked to a reduced risk of VTE, indicating that genetic profiling could play a significant role in improving risk assessment (22). The emergence of DOACs, like rivaroxaban and apixaban, offers potential benefits compared to traditional anticoagulants, such as LMWH. While emerging data suggest that DOACs may present a favorable safety profile for brain tumor patients, further studies are necessary to verify their effectiveness and safety within this population. Additionally, investigational anticoagulants that target factor XI are currently being examined for their ability to prevent thrombosis without significantly impacting hemostasis (19, 23). Identifying reliable biomarkers for assessing VTE risk in brain tumor patients remains an active area of research. Potential biomarkers, including TF, podoplanin, and circulating microparticles, are being explored for their ability to predict thrombotic events. The aim is to create a panel of biomarkers that can be used in conjunction with clinical risk factors to enhance the accuracy of VTE predictions (24).
Research in personalized medicine focuses on tailoring anticoagulation therapy to fit individual patient risk profiles. This approach involves using genetic and molecular data to inform treatment decisions, such as selecting the appropriate anticoagulant and determining the length of therapy. The objective of personalized treatment plans is to strike the right balance between reducing thrombotic risk and minimizing the likelihood of bleeding complications, especially intracerebral hemorrhages (25). Additionally, artificial intelligence (AI) holds great promise in enhancing our understanding of thrombosis risk. By meticulously analyzing extensive datasets that include patient demographics, detailed tumor characteristics, and a comprehensive range of biomarker profiles, AI can uncover valuable insights. This cutting-edge methodology enables more accurate identification of individuals at elevated risk for thrombosis, revolutionizing how healthcare professionals evaluate and manage these risks.
The present review had significant limitations. First, the heterogeneity across studies, such as variations in tumor types, grades, and anticoagulation regimens, prevented direct comparisons and meta-analysis. Second, the evidence base for DOACs in this population was limited, with few RCTs available. Additionally, potential publication bias might have affected our findings, as negative studies were often underrepresented, and excluding non-English literature might have omitted relevant data. Finally, the fast-evolving landscape of anticoagulant therapies and biomarkers highlights the need for timely reevaluation and standardized, prospective studies to improve thrombosis management in neuro-oncology.
Conclusions
The CAT presents significant challenges for patients with brain tumors influenced by tumor biology, treatment complications, and immobility. The diagnosis of CAT is difficult due to symptom overlap and bleeding risks. The LMWHs are the primary treatment, although DOACs and personalized approaches may improve outcomes. Effective management requires collaboration among oncologists, neurologists, and hematologists for risk assessments and tailored therapies. The present review summarizes current knowledge of CAT in brain tumor patients, emphasizing the need for more research into the efficacy and safety of DOACs, as well as demographic factors influencing VTE risk. A holistic, evidence-based approach is essential for minimizing thrombotic and bleeding complications while enhancing quality of life. In addition, future research and multidisciplinary collaborations are imperative for achieving these objectives.
Conflict of Interest
The authors declare that they have no competing interests.
Type of Study: Review |
Subject:
Neurosurgery
Received: 2025/03/15 | Accepted: 2025/05/27 | ePublished ahead of print: 2025/06/10 | Published: 2025/09/18
Received: 2025/03/15 | Accepted: 2025/05/27 | ePublished ahead of print: 2025/06/10 | Published: 2025/09/18
References
1. Jo JT, Schiff D, Perry JR. Thrombosis in brain tumors. Semin Thromb Hemost. 2014;40(3):325-31. [DOI:10.1055/s-0034-1370791]
2. Unruh D, Schwarze SR, Khoury L, et al. Tumor-derived tissue factor activates coagulation and enhances thrombosis in brain tumors. Nat Cancer. 2023;4(5):678-92.
3. Muster V, Gary T. Incidence, therapy, and bleeding risk-cancer-associated thrombosis in patients with glioblastoma. Cancers. 2020;12(6):1354. [DOI:10.3390/cancers12061354]
4. Khorana AA, Mackman N, Falanga A, Pabinger I, Noble S, Ageno W, et al. Cancer-associated venous thromboembolism. Nat Rev Dis Primers. 2022;8(1):11. [DOI:10.1038/s41572-022-00336-y]
5. Carney BJ, Wang TF, Al-Samkari H, et al. Management of cancer-associated thrombosis in patients with primary brain tumors: A systematic review and meta-analysis. J Thromb Haemost. 2024;22(1):112-25.
6. Diaz M, Jo J. Venous thrombotic events and anticoagulation in brain tumor patients. Curr Oncol Rep. 2022;24(4):493-500. [DOI:10.1007/s11912-021-01178-9]
7. Diaz M, Schiff D. Vascular complications in patients with brain tumors. Curr Opin Oncol. 2022;34(6):698-704. [DOI:10.1097/CCO.0000000000000875]
8. Greenberg SM, Ziai WC, Cordonnier C, et al. Anticoagulant selection for cancer-associated thrombosis in neuro-oncology: An international Delphi consensus. Neuro-Oncol Pract. 2023;10(4):389-401.
9. Magnus N, D'Asti E, Garnier D, Meehan B, Rak J. Brain neoplasms and coagulation. Semin Thromb Hemost. 2013;39(8):881-95. [DOI:10.1055/s-0033-1357483]
10. Campello E, Ilich A, Simioni P, Key NS. The role of microparticles in cancer-associated thrombosis: A focus on brain tumors. Blood Rev. 2025;54:100931.
11. Bierwagen M, Wierciński M, Góralczyk K, Góralczyk B, Janczarek A, Kulwas A, et al. Tissue factor-dependent coagulation activation in intracranial neoplasms: a comparative study. Blood Coagul Fibrinolysis. 2022;33(8):438-48. [DOI:10.1097/MBC.0000000000001164]
12. Riedl J, Preusser M, Nazari PM, Posch F, Panzer S, Marosi C, et al. Podoplanin expression in primary brain tumors induces platelet aggregation and increases risk of venous thromboembolism. Blood. 2017;129(13):1831-9. [DOI:10.1182/blood-2016-06-720714]
13. Perry JR. Thromboembolic disease in patients with high-grade glioma. Neuro-Oncol. 2012;14 (Suppl 4):iv73-80. [DOI:10.1093/neuonc/nos197]
14. Jo J, Diaz M, Horbinski C, Mackman N, Bagley S, Broekman M, et al. Epidemiology, biology, and management of venous thromboembolism in gliomas: an interdisciplinary review. Neuro-Oncol. 2023;25(8):1381-94. [DOI:10.1093/neuonc/noad059]
15. Leader A, Wilcox JA, Zwicker JI. How I treat acute venous thromboembolism in patients with brain tumors. Blood. 2024;144(17):1781-90. [DOI:10.1182/blood.2023023450]
16. Helfer H, Skaff Y, Happe F, Djennaoui S, Chidiac J, Poénou G, et al. Diagnostic approach for venous thromboembolism in cancer patients. Cancers. 2023;15(11):3031. [DOI:10.3390/cancers15113031]
17. Winther-Larsen A, Sandfeld-Paulsen B, Hvas AM. New insights in coagulation and fibrinolysis in patients with primary brain cancer: a systematic review. Semin Thromb Hemost. 2022;48(3):323-37. [DOI:10.1055/s-0041-1733961]
18. Swan D, Seiffge DJ, Thachil J. A review of anticoagulation in patients with central nervous system malignancy: between a rock and a hard place. J Neurol. 2021;268(7):2390-2401. [DOI:10.1007/s00415-020-09775-7]
19. Swartz AW, Drappatz J. Safety of direct oral anticoagulants in central nervous system malignancies. Oncologist. 2021;26(5):427-32. [DOI:10.1002/onco.13698]
20. Wilhelmy F, Gaier M, Planitzer U, Kasper J, prasse G, Frydrychowicz C, et al. Venous thromboembolism and intracranial hemorrhage in patients undergoing glioblastoma surgery. Sci Rep. 2023;13(1):21679. [DOI:10.1038/s41598-023-48542-2]
21. Bailey D, Wilding H, Ganesalingam N, Rizk E. Perioperative management of antiplatelet and anticoagulation in brain tumor surgery: a survey of international practices. World Neurosurg. 2024;190:e271-e280. [DOI:10.1016/j.wneu.2024.07.111]
22. Riedl J, Ay C. Venous thromboembolism in brain tumors: risk factors, molecular mechanisms, and clinical challenges. Semin Thromb Hemost. 2019;45(4):334-41. [DOI:10.1055/s-0039-1688493]
23. Iyengar V, Patell R, Zwicker J. Challenges in anticoagulation for patients with brain tumors. Best Pract Res Clin Haematol. 2022;35(1):101350. [DOI:10.1016/j.beha.2022.101350]
24. Sartori MT, Della Puppa A, Ballin A, Campello E, Radu CM, Saggiorato G, et al. Circulating microparticles of glial origin and tissue factor bearing in high-grade glioma: a potential prothrombotic role. Thromb Haemost. 2013;110(2):378-85. [DOI:10.1160/TH12-12-0957]
25. Leader A, Wilcox JA, Zwicker JI. How I treat acute venous thromboembolism in patients with brain tumors. Blood. 2024;144(17):17 81-90. [DOI:10.1182/blood.2023023450]
Send email to the article author
| Rights and permissions | |
 |
This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License. |








