Volume 12, Issue 4 (12-2024)
J Surg Trauma 2024, 12(4): 129-136 |
Back to browse issues page
Download citation:
BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:



BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:
Ebrahimi Shah-abadi M, Heydari M, Najmaddini M, Soltani E. Outcomes of Hemostatic Management in Hemophilia Patients without Inhibitors Undergoing Invasive or Surgical Procedures: A Systemic Review and Meta-analysis. J Surg Trauma 2024; 12 (4) :129-136
URL: http://jsurgery.bums.ac.ir/article-1-386-en.html
URL: http://jsurgery.bums.ac.ir/article-1-386-en.html
Assistant fellowship of Surgical Oncology, Mashhad University of Medical Sciences, Mashhad, Iran
Full-Text [PDF 561 kb]
(386 Downloads)
| Abstract (HTML) (1448 Views)
Abstract
Introduction: There are numerous questions surrounding the use, duration of administration, and dosage of clotting factor concentrates (CFC). This issue has been so far very controversial and challenging. Given the critical importance of the subject, the present study aimed to assess the outcomes of perioperative hemostatic management in hemophilia patients without inhibitors undergoing invasive or surgical procedures.
Methods: All articles published in international databases, such as PubMed, Scopus, Science Direct, and Embase, until May 2022 were included. Data analysis was performed using STATA software (version 16).
Results: The search in databases yielded 1,218 articles and the full text of 192 articles was reviewed. Finally, nine articles that met the inclusion criteria entered the analysis. Mean differences of bleeding rate between high-dose prophylaxis and episodic groups was -53.40 (MD; 95 CI (-53.72, -53.08); P=0.01). The mean differences of joint bleeding rate between intermediate dose prophylaxis and episodic groups was -12.79 (MD; 95 CI (-12.85, -12.74); P=0.01).
Conclusion: Based on the present meta-analysis, it was revealed that in patients with hemophilia A, the use of prophylaxis has better results in terms of annualized bleeding rate and annualized joint bleeding rate than episodic treatment.
Key words: Bleeding, Disease management, Hemophilia, Hemostatics
I2 index test was used to evaluate the level of heterogeneity (I2< 50% = low levels, 502< 75% = moderate and I2>75% = high levels). 95% confidence interval on risk ratio and mean differences between episodic vs prophylactic treatment about bleeding rate were performed with fixed effect model and in-variance and Mantel-Haenszel method. Data analysis was carried out using STATA software (version 16).
Results
The database searches yielded 1,218 articles. After importing all articles into EndNote.X8 software, duplicate articles were deleted (n=181). Thereafter, 1,037 articles were entered and examined in the second stage. At this stage, while reviewing the titles and abstracts of articles, 845 unrelated articles were excluded from the study. In the third stage, the full text of 192 articles was reviewed. Finally, nine articles that were published until May 2022 and met the inclusion criteria entered the analysis (Figure 1).
The number of patients with hemophilia in the intervention (prophylaxis) and control groups (episodic) were 311 and 272, respectively. Their mean age was 23.41 years (Table2).
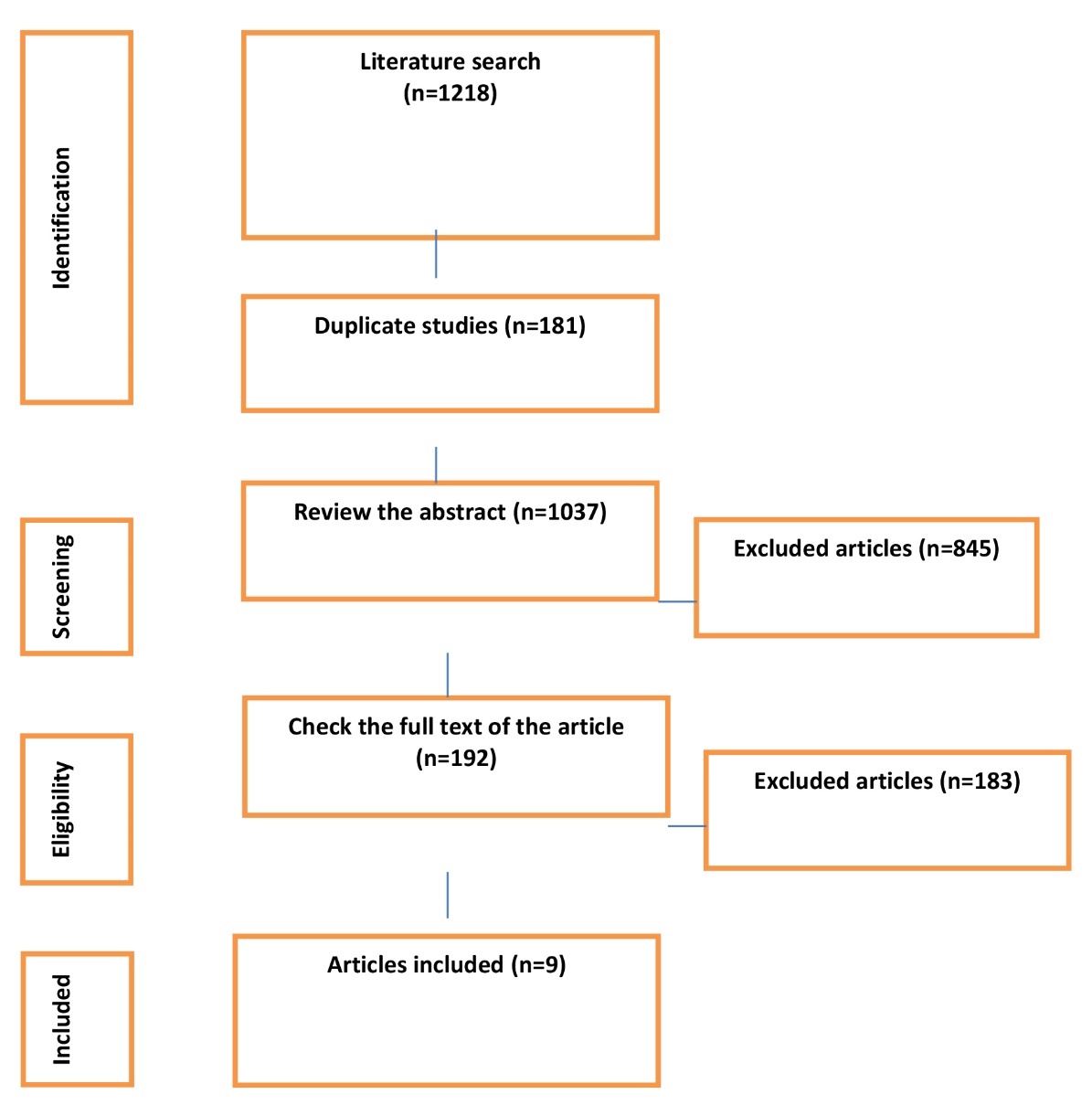
Table 2. Summary of studies characteristics

Full-Text: (391 Views)
Abstract
Introduction: There are numerous questions surrounding the use, duration of administration, and dosage of clotting factor concentrates (CFC). This issue has been so far very controversial and challenging. Given the critical importance of the subject, the present study aimed to assess the outcomes of perioperative hemostatic management in hemophilia patients without inhibitors undergoing invasive or surgical procedures.
Methods: All articles published in international databases, such as PubMed, Scopus, Science Direct, and Embase, until May 2022 were included. Data analysis was performed using STATA software (version 16).
Results: The search in databases yielded 1,218 articles and the full text of 192 articles was reviewed. Finally, nine articles that met the inclusion criteria entered the analysis. Mean differences of bleeding rate between high-dose prophylaxis and episodic groups was -53.40 (MD; 95 CI (-53.72, -53.08); P=0.01). The mean differences of joint bleeding rate between intermediate dose prophylaxis and episodic groups was -12.79 (MD; 95 CI (-12.85, -12.74); P=0.01).
Conclusion: Based on the present meta-analysis, it was revealed that in patients with hemophilia A, the use of prophylaxis has better results in terms of annualized bleeding rate and annualized joint bleeding rate than episodic treatment.
Key words: Bleeding, Disease management, Hemophilia, Hemostatics
Introduction
Hemophilia is a genetic bleeding disorder, and the patients suffering from this disease experience prolonged bleeding due to coagulation factor deficiency (1). In hemophilia A, or classical hemophilia, the body is unable to produce coagulation factor VIII (2). Hemophilia B is caused by the dysfunction of coagulation factor IX (3). This disease is treated by injecting a drug containing a non-existent coagulation factor into a vein. In some people with hemophilia, this factor is recognized by the body as a foreign protein, and the body produces an antibody (inhibitor) that kills the factor (4). In this way, these people become resistant to treatment.
When a person with hemophilia develops an inhibitor, they are treated to remove antibodies (immune tolerance induction) and for acute episodes of bleeding (5). The most challenging issue for surgeons and hematologists is the intraoperative management of hemophilia patients. According to reports, the mortality rate of the patients undergoing surgery is about 60% (6-8). Studies have demonstrated that the improvement of postoperative clotting factor concentrates (CFC) reduces mortality rate by about 4.5%-5% (9, 10). Prophylactic therapies of bleeding and episodic invoicing, which are very expensive, are used in the treatment of these patients (11).
Prophylactic therapy defined as the administration of a factor in the absence of bleeding is a treatment strategy used to reduce bleeding (12). Clotting factor concentrate prophylaxis aims to preserve joint function by converting severe hemophilia (factor VIII or IX less than 1%) into a clinically milder form of the disease (13). Surgery in hemophilia patients carries the risk of bleeding, postoperative infections, and wound healing (14, 15). Common postoperative complications are mainly due to inadequate CFC administration. CFCs are more extensively available in developed countries. Studies have pointed out that CFCs are highly effective in the intraoperative management of people with hemophilia who have undergone invasive or surgical procedures (6, 16).
In 2020, World Hemophilia Federation reported that it is important to study episodic therapy and prophylactic therapy and compare the two methods (17). There are numerous questions about use, duration of administration, and dosage of CFC, and this issue has been so far very controversial and challenging. Given the critical importance of this subject, the present study aimed to assess the outcomes of perioperative hemostatic management in hemophilia patients without inhibitors undergoing invasive or surgical procedures.
Methods
The present research is a systematic review and meta-analysis study based on PRISMA guidelines (18). A query was conducted on PubMed, Scopus, Science Direct, and Embase databases until May 2022 using the following keywords:
(((("Hemophilia A"[Mesh] OR "Hemophilia B"[Mesh] OR "Factor XI Deficiency"[Mesh] OR "von Willebrand Diseases"[Mesh] OR "Hemophilia A with Vascular Abnormality" [Supplementary Concept] OR "Factor 8 deficiency, acquired" [Supplementary Concept] OR "F8 protein, human" [Supplementary Concept]) AND ( "Gastroenterostomy"[Mesh] OR "Bariatric Surgery"[Mesh] )) AND "Minor
Surgical Procedures"[Mesh]) AND ( "Cardiac Catheters" [Mesh] OR "Cardiac Surgical Procedures" [Mesh] OR "Cardiac Catheterization"[Mesh] )) AND (“Hemostatics" [Mesh] OR "Hemostatics" [Pharmacological Action] OR “Hemostatic Techniques"[Mesh] ).
The inclusion criteria entailed randomized controlled trials studies, different factor replacement therapies, and types of clotting factor concentrates. On the other hand, the exclusion criteria were the management of hemophilia patients with an inherited or acquired hemostatic defect other than hemophilia, history of inhibitors, low platelet count, and diagnosis of cirrhosis. The PECO (participants, exposure, comparison, outcome) strategy was used to answer the research questions (Table 1).
Table 1. PECO strategy
Hemophilia is a genetic bleeding disorder, and the patients suffering from this disease experience prolonged bleeding due to coagulation factor deficiency (1). In hemophilia A, or classical hemophilia, the body is unable to produce coagulation factor VIII (2). Hemophilia B is caused by the dysfunction of coagulation factor IX (3). This disease is treated by injecting a drug containing a non-existent coagulation factor into a vein. In some people with hemophilia, this factor is recognized by the body as a foreign protein, and the body produces an antibody (inhibitor) that kills the factor (4). In this way, these people become resistant to treatment.
When a person with hemophilia develops an inhibitor, they are treated to remove antibodies (immune tolerance induction) and for acute episodes of bleeding (5). The most challenging issue for surgeons and hematologists is the intraoperative management of hemophilia patients. According to reports, the mortality rate of the patients undergoing surgery is about 60% (6-8). Studies have demonstrated that the improvement of postoperative clotting factor concentrates (CFC) reduces mortality rate by about 4.5%-5% (9, 10). Prophylactic therapies of bleeding and episodic invoicing, which are very expensive, are used in the treatment of these patients (11).
Prophylactic therapy defined as the administration of a factor in the absence of bleeding is a treatment strategy used to reduce bleeding (12). Clotting factor concentrate prophylaxis aims to preserve joint function by converting severe hemophilia (factor VIII or IX less than 1%) into a clinically milder form of the disease (13). Surgery in hemophilia patients carries the risk of bleeding, postoperative infections, and wound healing (14, 15). Common postoperative complications are mainly due to inadequate CFC administration. CFCs are more extensively available in developed countries. Studies have pointed out that CFCs are highly effective in the intraoperative management of people with hemophilia who have undergone invasive or surgical procedures (6, 16).
In 2020, World Hemophilia Federation reported that it is important to study episodic therapy and prophylactic therapy and compare the two methods (17). There are numerous questions about use, duration of administration, and dosage of CFC, and this issue has been so far very controversial and challenging. Given the critical importance of this subject, the present study aimed to assess the outcomes of perioperative hemostatic management in hemophilia patients without inhibitors undergoing invasive or surgical procedures.
Methods
The present research is a systematic review and meta-analysis study based on PRISMA guidelines (18). A query was conducted on PubMed, Scopus, Science Direct, and Embase databases until May 2022 using the following keywords:
(((("Hemophilia A"[Mesh] OR "Hemophilia B"[Mesh] OR "Factor XI Deficiency"[Mesh] OR "von Willebrand Diseases"[Mesh] OR "Hemophilia A with Vascular Abnormality" [Supplementary Concept] OR "Factor 8 deficiency, acquired" [Supplementary Concept] OR "F8 protein, human" [Supplementary Concept]) AND ( "Gastroenterostomy"[Mesh] OR "Bariatric Surgery"[Mesh] )) AND "Minor
Surgical Procedures"[Mesh]) AND ( "Cardiac Catheters" [Mesh] OR "Cardiac Surgical Procedures" [Mesh] OR "Cardiac Catheterization"[Mesh] )) AND (“Hemostatics" [Mesh] OR "Hemostatics" [Pharmacological Action] OR “Hemostatic Techniques"[Mesh] ).
The inclusion criteria entailed randomized controlled trials studies, different factor replacement therapies, and types of clotting factor concentrates. On the other hand, the exclusion criteria were the management of hemophilia patients with an inherited or acquired hemostatic defect other than hemophilia, history of inhibitors, low platelet count, and diagnosis of cirrhosis. The PECO (participants, exposure, comparison, outcome) strategy was used to answer the research questions (Table 1).
Table 1. PECO strategy
| PECO strategy | Description |
| P | Population: patients with hemophilia |
| E | Exposure: invasive or surgical procedures |
| C | Comparison: Episodic vs prophylactic treatment |
| O | Outcome: bleeding rate |
I2 index test was used to evaluate the level of heterogeneity (I2< 50% = low levels, 50
Results
The database searches yielded 1,218 articles. After importing all articles into EndNote.X8 software, duplicate articles were deleted (n=181). Thereafter, 1,037 articles were entered and examined in the second stage. At this stage, while reviewing the titles and abstracts of articles, 845 unrelated articles were excluded from the study. In the third stage, the full text of 192 articles was reviewed. Finally, nine articles that were published until May 2022 and met the inclusion criteria entered the analysis (Figure 1).
The number of patients with hemophilia in the intervention (prophylaxis) and control groups (episodic) were 311 and 272, respectively. Their mean age was 23.41 years (Table2).

Figure 1. PRISMA flowcharts
Table 2. Summary of studies characteristics
| Study. Years | Hemophilia type | Sample size | Mean of age (years) | Follow-up period (months) | Result | |
| Intervention | control | |||||
| Chozie et al., 2019 (19) | A | 25 | 25 | 11.95 | 11.95 | Improvement was observed at the sixth month among patients in the prophylaxis group. |
| Manco‐Johnson et al., 2017 (20) | A | 41 | 42 | 29 | 29 | A reduction in joint bleeding events was noted in the prophylaxis group. |
| Verma et al., 2016 (21) | A | 11 | 10 | 6.11 | 6.11 | The prophylaxis group did not experience any significant complications |
| Kavakli et al., 2015 (22) | A | 59 | 21 | 29.6 | 29.6 | The administration of BAY 81-8973, a full-length, plasma protein-free recombinant factor VIII product, was well tolerated and resulted in a reduction in the median annualized bleeding rate (ABR). |
| Valentino et al., 2014 (23) | B | 25 | 22 | 28.4 | 28.4 | Both prophylaxis treatments showed a favorable safety profile in patients with hemophilia B. |
| Valentino et al., 2012 (24) | A | 34 | 32 | 27.5 | 27.5 | No significant differences were observed in Factor VIII consumption or adverse event rates across the prophylaxis regimens. |
| Powell et al., 2012 (25) | A | 63 | 68 | 33.6 | 33.6 | A significant difference in efficacy was noted between the treatment groups, with the rFVIII-FS control group showing fewer than 9 bleeds per year, compared to the subjects treated with BAY 79-4980. |
| Gringeri et al., 2011 (26) | A | 21 | 19 | 4.10 | 4.10 | Prophylaxis demonstrated greater effectiveness when initiated early (at or before 36 months), resulting in fewer joint bleeds among patients. |
| Manco‐Johnson et al.,2007 (27) | A | 32 | 33 | 1.6 | 1.6 | High titers of factor VIII inhibitors developed in two boys undergoing prophylaxis, while three boys in the episodic-therapy group experienced life-threatening hemorrhages. Hospitalizations and infections related to central-catheter placement showed no significant differences between the two groups. |
Annualized bleeding rate (ABR)
Subgroup meta-analysis
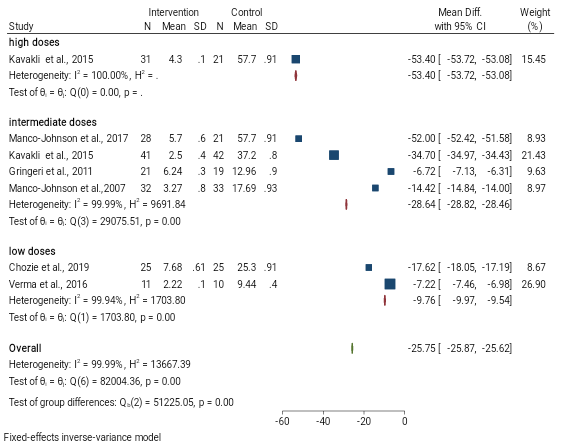
The mean differences of bleeding rate between high-dose prophylaxis and episodic groups was -53.40 (MD; 95 CI (-53.72, -53.08); P=0.01). As illustrated in Figure 2, the mean bleeding rate was lower in the high-dose prophylaxis group than the episodic group. The mean differences of bleeding rate between intermediate dose prophylaxis and episodic groups was -28.34 (MD; 95 CI (-28.82, -28.46); P=0.01). According to Figure 2, the mean bleeding rate was lower in the intermediate-dose prophylaxis group than the episodic group.
Mean differences of Bleeding rate between low dose prophylaxis and episodic groups was -9.76 (MD; 95 CI (-9.97, -9.54); P=0.01). As presented in Figure 2, the mean bleeding rate was lower in the low-dose prophylaxis group than the episodic group. According to the test of group differences, the difference observed between the groups (P=0.00) and high heterogeneity (I2=99%; P=0.00) was among the numerous studies which indicate it could not provide sufficient evidence.
Annualized joint bleeding rate (AJBR)
Subgroup meta-analysis
The mean differences of joint bleeding rate between high-dose prophylaxis and episodic groups was -40.30 (MD; 95 CI -40.40, -40.11); P=0.01). As displayed in Figure 3, the mean joint bleeding rate was lower in high-dose prophylaxis group than episodic group. The mean differences of joint bleeding rate between intermediate dose prophylaxis and episodic groups was -12.79 (MD; 95 CI (-12.85, -12.74); P=0.01). According to Figure 3, the mean of joint bleeding rate was lower in intermediate-dose prophylaxis group than episodic group.
The mean differences of joint bleeding rate between low dose prophylaxis and episodic groups was -6.81 (MD; 95 CI (-6.68, -6.75); P=0.01). According to Figure 3, the mean joint bleeding rate was lower in low-dose prophylaxis group than episodic group. According to test of group differences, the difference observed between the groups (P=0.00) and high heterogeneity (I2=100%; p=0.00) was among the numerous studies which indicate it could not provide sufficient evidence.
Subgroup meta-analysis
The mean differences of bleeding rate between high-dose prophylaxis and episodic groups was -53.40 (MD; 95 CI (-53.72, -53.08); P=0.01). As illustrated in Figure 2, the mean bleeding rate was lower in the high-dose prophylaxis group than the episodic group. The mean differences of bleeding rate between intermediate dose prophylaxis and episodic groups was -28.34 (MD; 95 CI (-28.82, -28.46); P=0.01). According to Figure 2, the mean bleeding rate was lower in the intermediate-dose prophylaxis group than the episodic group.
Mean differences of Bleeding rate between low dose prophylaxis and episodic groups was -9.76 (MD; 95 CI (-9.97, -9.54); P=0.01). As presented in Figure 2, the mean bleeding rate was lower in the low-dose prophylaxis group than the episodic group. According to the test of group differences, the difference observed between the groups (P=0.00) and high heterogeneity (I2=99%; P=0.00) was among the numerous studies which indicate it could not provide sufficient evidence.
Annualized joint bleeding rate (AJBR)
Subgroup meta-analysis
The mean differences of joint bleeding rate between high-dose prophylaxis and episodic groups was -40.30 (MD; 95 CI -40.40, -40.11); P=0.01). As displayed in Figure 3, the mean joint bleeding rate was lower in high-dose prophylaxis group than episodic group. The mean differences of joint bleeding rate between intermediate dose prophylaxis and episodic groups was -12.79 (MD; 95 CI (-12.85, -12.74); P=0.01). According to Figure 3, the mean of joint bleeding rate was lower in intermediate-dose prophylaxis group than episodic group.
The mean differences of joint bleeding rate between low dose prophylaxis and episodic groups was -6.81 (MD; 95 CI (-6.68, -6.75); P=0.01). According to Figure 3, the mean joint bleeding rate was lower in low-dose prophylaxis group than episodic group. According to test of group differences, the difference observed between the groups (P=0.00) and high heterogeneity (I2=100%; p=0.00) was among the numerous studies which indicate it could not provide sufficient evidence.

Figure 2. Forest plot illustrating bleeding rate between prophylaxis and episodic group

Figure 3. Forest plot demonstrating joint bleeding rate between prophylaxis and episodic groups
Discussion
The present study aimed to assess prophylactic therapies in comparison with an episodic treatment. As evidenced by the findings of the present study, prophylactic treatments are more effective and have more benefits. High-dose prophylactic anticoagulation of ABR had a better dose-response effect. Compared to other doses, it was revealed that the doses had a more marked effect on ABR and not on AJBR. It is worth noting that smaller sample size and smaller number of joint hemorrhages should be considered and these results should be examined more sensitively. According to repeated and comprehensive searches, only one study has compared high dose and prophylaxis against episodic treatment that did not have a large sample size. The selected studies in the present research were performed in different countries where different CFCs were used according to different health systems. No study has examined different doses of prophylaxis treatment and the effect of doses. Therefore, in order to provide sufficient evidence and strong results, more studies are needed in this field. Based on studies in patients with hemophilia, the use of prophylaxis with moderate and low doses yielded better results in terms of lower ABR and lower AJBR than episodic treatment (28, 29). Non-randomized studies examining different doses demonstrated lower ABR and lower AJBR in the moderate dose group (30, 31). A study of high and moderate CFC doses pointed out that AJBR was less common in high-dose patients (32, 33). Based on the results of I2, it was found that there is a high heterogeneity between the findings of the studies and future research should employ a similar method so that the reported means of the studies are similar since in the studies selected for the present meta-analysis, the means reported for ABR and AJBR were very different. On the other hand, the CFCs used in the studies had different characteristics. In some studies, plasma-derived products were used, while in some others, recombinant concentrates were used, all with standard half-lives. According to the World Federation of Hemophilia Guidelines 2020(34), the use of these two types of CFCs is a good treatment for people with hemophilia since evidence has suggested that both methods are safe for the treatment and prevention of bleeding and their positive effects are evident (35). Nonetheless, there is a need for further studies in this area to examine the types of CFCs with different characteristics. Future studies need to be well-designed to achieve comprehensive results and provide stronger evidence.
The follow-up period was very different, and studies need to be designed with the same follow-up periods. The sample size of the studies was very small and studies with larger sample sizes are needed. Moreover, the studies used a variety of alternative therapies that should be well-designed for future studies. Almost all studies have been performed on patients with hemophilia A, and mostly on the age group of children. Future studies need to be performed on patients with hemophilia B and the adult age group. The means reported for ABR and AJBR findings were so different that the working methodology of future studies should be well considered. In addition, the quality of the studies was moderate to low and future studies should be well designed (blinding participants, large sample size, follow-up period, blinding the researcher, and reporting findings). Based on the above limitations, great caution needs to be exercised in the generalizations of the results of this study to the age group of adults and patients with hemophilia B.
Conclusions
Based on the present meta-analysis, it was revealed that in people with hemophilia A, the use of prophylaxis has better results in terms of ABR and AJBR than episodic treatment. Nevertheless, due to the small sample size and variations in the types of alternative treatments with CFC, more RCT studies are needed to confirm the available and sufficient evidence. Higher-quality studies are needed to assist in the decision-making process regarding the use of alternative therapies.
Conflict of Interest
The authors declare that they have no conflict of interest.
The present study aimed to assess prophylactic therapies in comparison with an episodic treatment. As evidenced by the findings of the present study, prophylactic treatments are more effective and have more benefits. High-dose prophylactic anticoagulation of ABR had a better dose-response effect. Compared to other doses, it was revealed that the doses had a more marked effect on ABR and not on AJBR. It is worth noting that smaller sample size and smaller number of joint hemorrhages should be considered and these results should be examined more sensitively. According to repeated and comprehensive searches, only one study has compared high dose and prophylaxis against episodic treatment that did not have a large sample size. The selected studies in the present research were performed in different countries where different CFCs were used according to different health systems. No study has examined different doses of prophylaxis treatment and the effect of doses. Therefore, in order to provide sufficient evidence and strong results, more studies are needed in this field. Based on studies in patients with hemophilia, the use of prophylaxis with moderate and low doses yielded better results in terms of lower ABR and lower AJBR than episodic treatment (28, 29). Non-randomized studies examining different doses demonstrated lower ABR and lower AJBR in the moderate dose group (30, 31). A study of high and moderate CFC doses pointed out that AJBR was less common in high-dose patients (32, 33). Based on the results of I2, it was found that there is a high heterogeneity between the findings of the studies and future research should employ a similar method so that the reported means of the studies are similar since in the studies selected for the present meta-analysis, the means reported for ABR and AJBR were very different. On the other hand, the CFCs used in the studies had different characteristics. In some studies, plasma-derived products were used, while in some others, recombinant concentrates were used, all with standard half-lives. According to the World Federation of Hemophilia Guidelines 2020(34), the use of these two types of CFCs is a good treatment for people with hemophilia since evidence has suggested that both methods are safe for the treatment and prevention of bleeding and their positive effects are evident (35). Nonetheless, there is a need for further studies in this area to examine the types of CFCs with different characteristics. Future studies need to be well-designed to achieve comprehensive results and provide stronger evidence.
The follow-up period was very different, and studies need to be designed with the same follow-up periods. The sample size of the studies was very small and studies with larger sample sizes are needed. Moreover, the studies used a variety of alternative therapies that should be well-designed for future studies. Almost all studies have been performed on patients with hemophilia A, and mostly on the age group of children. Future studies need to be performed on patients with hemophilia B and the adult age group. The means reported for ABR and AJBR findings were so different that the working methodology of future studies should be well considered. In addition, the quality of the studies was moderate to low and future studies should be well designed (blinding participants, large sample size, follow-up period, blinding the researcher, and reporting findings). Based on the above limitations, great caution needs to be exercised in the generalizations of the results of this study to the age group of adults and patients with hemophilia B.
Conclusions
Based on the present meta-analysis, it was revealed that in people with hemophilia A, the use of prophylaxis has better results in terms of ABR and AJBR than episodic treatment. Nevertheless, due to the small sample size and variations in the types of alternative treatments with CFC, more RCT studies are needed to confirm the available and sufficient evidence. Higher-quality studies are needed to assist in the decision-making process regarding the use of alternative therapies.
Conflict of Interest
The authors declare that they have no conflict of interest.
Type of Study: Meta-analysis |
Subject:
General Surgery
Received: 2023/06/23 | Accepted: 2024/12/15 | ePublished ahead of print: 2024/12/21 | Published: 2024/12/30
Received: 2023/06/23 | Accepted: 2024/12/15 | ePublished ahead of print: 2024/12/21 | Published: 2024/12/30
References
1. Castaman G, Matino D. Hemophilia A and B: molecular and clinical similarities and differences. Haematologica. 2019;104(9):1702. [DOI:10.3324/haematol.2019.221093]
2. Knöbl P. Prevention and management of bleeding episodes in patients with acquired hemophilia A. Drugs. 2018;78(18):1861-1872. [DOI:10.1007/s40265-018-1027-y]
3. Cooley B, Broze Jr GJ, Mann DM, Lin F-C, Pedersen LG, Stafford DW. Dysfunctional endogenous FIX impairs prophylaxis in a mouse hemophilia B model. Blood. 2019;133(22):2445-2451. [DOI:10.1182/blood.2018884015]
4. Matino D, Makris M, Dwan K, D'Amico R, Iorio A. Recombinant Factor VIIa concentrate versus plasma derived concentrates for the treatment of acute bleeding episodes in people with haemophilia and inhibitors. Cochrane Database Syst Rev. 2015;2015(12):CD004449. [DOI:10.1002/14651858.CD004449.pub4]
5. Paisley S, Wight J, Currie E, Knight C. The management of inhibitors in haemophilia A: introduction and systematic review of current practice. Haemophilia. 2003;9(4):405-417. [DOI:10.1046/j.1365-2516.2003.00779.x]
6. Tong K-M, Wang J-D, Chang S-T, Cheng Y-Y, Wang S-S. Outcome of perioperative hemostatic management in patients with hemophilia without inhibitors undergoing 161 invasive or surgical procedures. J Chin Med Assoc. 2018;81(10):926-929. [DOI:10.1016/j.jcma.2017.11.016]
7. Abou-Ismail MY, Connell NT. How to manage bleeding disorders in aging patients needing surgery. Hematology. 2021;2021(1):529-535. [DOI:10.1182/hematology.2021000288]
8. Chen X, Miao Q, Zhu T, Zhang C. Replacement Therapy for Hemophilia Patients Undergoing Cardiac Surgery: Report of Three Cases. Chin Med Sci J. 2022;37(1):79-81. [DOI:10.24920/003929]
9. Dhal B, Dutta A, Buragohain M, Rajan A. Fracture Shaft of Femur in PwH (person with hemophilia): Surgery or conservative? Assam J Internal Med. 2021;11(1):40-44 [DOI:10.4103/2278-8239.346809]
10. Rudowski W. Moynihan Lecture, 1980. Major surgery in haemophilia. Ann R Coll Surg Engl. 1981;63(2):111.
11. Smith PS, Teutsch SM, Shaffer PA, Rolka H, Evatt B. Episodic versus prophylactic infusions for hemophilia A: a cost-effectiveness analysis. J Pediatr. 1996;129(3):424-431. [DOI:10.1016/S0022-3476(96)70076-8]
12. Ljung R, Auerswald G, Benson G, Dolan G, Duffy A, Hermans C, et al. Inhibitors in haemophilia A and B: Management of bleeds, inhibitor eradication and strategies for difficult‐to‐treat patients. Eur J Haematol. 2019;102(2):111-122. [DOI:10.1111/ejh.13193]
13. Iorio A, Marchesini E, Marcucci M, Stobart K, Chan AK. Clotting factor concentrates given to prevent bleeding and bleeding‐related complications in people with hemophilia A or B. Cochrane Database Syst Rev. 2011:(9):CD003429. [DOI:10.1002/14651858.CD003429.pub4]
14. Rodriguez‐Merchan E. Surgical wound healing in bleeding disorders. Haemophilia. 2012;18(4):487-490. [DOI:10.1111/j.1365-2516.2012.02760.x]
15. Rodriguez-Merchan EC. Musculoskeletal complications of hemophilia. HSS J. 2010;6(1):37-42. [DOI:10.1007/s11420-009-9140-9]
16. Srivastava A, Santagostino E, Dougall A, Kitchen S, Sutherland M, Pipe SW, et al. WFH guidelines for the management of hemophilia. Haemophilia. 2020; 26 Suppl 6:1-158. [DOI:10.1111/hae.14046]
17. Delgado-Flores CJ, García-Gomero D, Salvador-Salvador S, Montes-Alvis J, et al . Effects of replacement therapies with clotting factors in patients with hemophilia: A systematic review and meta-analysis. PloS One. 2022;17(1): e0262273. [DOI:10.1371/journal.pone.0262273]
18. Wu Y-C, Chen C-S, Chan Y-J. The outbreak of COVID-19: An overview. J Chin Med Assoc. 2020;83(3):217-220. [DOI:10.1097/JCMA.0000000000000270]
19. Chozie NA, Primacakti F, Gatot D, Setiabudhy RD, Tulaar AB, Prasetyo M. Comparison of the efficacy and safety of 12‐month low‐dose factor VIII tertiary prophylaxis vs on‐demand treatment in severe haemophilia A children. Haemophilia. 2019;25(4):633-639. [DOI:10.1111/hae.13770]
20. Manco‐Johnson MJ, Lundin B, Funk S, Peterfy C, Raunig D, Werk M, et al. Effect of late prophylaxis in hemophilia on joint status: a randomized trial. J Thromb Haemost. 2017;15(11):2115-2124. [DOI:10.1111/jth.13811]
21. Verma S, Dutta T, Mahadevan S, Nalini P, Basu D, Biswal N, et al. A randomized study of very low‐dose factor VIII prophylaxis in severe haemophilia-A success story from a resource limited country. Haemophilia. 2016;22(3):342-348. [DOI:10.1111/hae.12838]
22. Kavakli K, Yang R, Rusen L, Beckmann H, Tseneklidou‐Stoeter D, Maas Enriquez M, et al. Prophylaxis vs. on‐demand treatment with BAY 81‐8973, a full‐length plasma protein‐free recombinant factor VIII product: results from a randomized trial (LEOPOLD II). J Thromb Haemost. 2015;13(3):360-369. [DOI:10.1111/jth.12828]
23. Valentino L, Rusen L, Elezovic I, Smith L, Korth‐Bradley J, Rendo P. Multicentre, randomized, open‐label study of on‐demand treatment with two prophylaxis regimens of recombinant coagulation factor IX in haemophilia B subjects. Haemophilia. 2014;20(3):398-406. [DOI:10.1111/hae.12344]
24. Valentino L, Mamonov V, Hellmann A, Quon D, Chybicka A, Schroth P, et al. A randomized comparison of two prophylaxis regimens and a paired comparison of on‐demand and prophylaxis treatments in hemophilia A management. J Thromb Haemost. 2012;10(3):359-367. [DOI:10.1111/j.1538-7836.2011.04611.x]
25. Powell J, Martinowitz U, Windyga J, Di Minno G, Hellmann A, Pabinger I, et al. Efficacy and safety of prophylaxis with once-weekly BAY 79-4980 compared with thrice-weekly rFVIII-FS in haemophilia A patients. Thromb Haemost. 2012;108(11):913-922. [DOI:10.1160/TH12-03-0188]
26. Gringeri A, Lundin B, Von Mackensen S, Mantovani L, Mannucci P, Group ES. A randomized clinical trial of prophylaxis in children with hemophilia A (the ESPRIT Study). J Thromb Haemost. 2011;9(4):700-710. [DOI:10.1111/j.1538-7836.2011.04214.x]
27. Manco-Johnson M, Abshire T, Shapiro A. Recombinant factor VIII for the prevention of joint disease in children with severe hemophilia: prophylaxis compared with episodic treatment. N Engl J Med. 2007;357(6):535-544. [DOI:10.1056/NEJMoa067659]
28. Zhao Y, Xiao J, Yang R, Wu R, Hu Y, Beckmann H, et al. Efficacy of standard prophylaxis versus on-demand treatment with Bayer's sucrose-formulated recombinant FVIII (rFVIII-FS) in Chinese children with severe hemophilia A. Pediatr Hematol Oncol. 2017;34(3):138-148. [DOI:10.1080/08880018.2017.1313921]
29. Wu R, Luke KH, Poon MC, Wu X, Zhang N, Zhao L, et al. Low dose secondary prophylaxis reduces joint bleeding in severe and moderate haemophilic children: a pilot study in China. Haemophilia. 2011;17(1):70-74. [DOI:10.1111/j.1365-2516.2010.02348.x]
30. Qiu S-Q, Zhuang J-M, Zhou X, Yin R-X, Liu Z-Q, Ma F, et al. Breakthrough bleeding in adult patients with severe hemophilia A receiving low-and intermediate-dose FVIII for tertiary prophylaxis: characteristics and influencing factors. Nan Fang Yi Ke Da Xue Xue Bao. 2017;37(10):1391-1395.
31. Zhuang J-M, Sun X-Y, Zhou X, Liu Z-Q, Sun J. Prophylactic treatment with low-and intermediate-dose factor VIII in children with severe hemophilia A: comprehensive evaluation of joint outcomes and correlation analysis. Nan Fang Yi Ke Da Xue Xue Bao. 2018;38(4):496-501.
32. Fischer K, Steen Carlsson K, Petrini P, Holmström M, Ljung R, van den Berg HM, et al. Intermediate-dose versus high-dose prophylaxis for severe hemophilia: comparing outcome and costs since the 1970s. Blood. 2013;122(7):1129-1136. [DOI:10.1182/blood-2012-12-470898]
33. Wang Y, Yang Q, Zheng L, Wang X, Jiang W, Lu L, et al. Efficacy of Individualized Preventive Treatment of Patients with Severe Hemophilia A Guided by Multiple Clinical Parameters and Pharmacokinetics. Acta Haematol. 2022;145(4):354-361. [DOI:10.1159/000521360]
34. Hermans C, Makris M. 'Haemophilia Guidelines for All': A new ambition of the World Federation of Haemophilia (WFH). Haemophilia. 2020;26(5):748-749. [DOI:10.1111/hae.14093]
35. Srivastava A, Brewer A, Mauser‐Bunschoten E, Key N, Kitchen S, Llinas A, et al. Guidelines for the management of hemophilia. Haemophilia. 2013;19(1): e1-e47. [DOI:10.1111/j.1365-2516.2012.02909.x]
Send email to the article author
| Rights and permissions | |
 |
This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License. |








